Traumatic Brain Injury: Evaluation, Treatment, and Rehabilitation
Novartis Kisqali NATALEE Analysis Reinforces Consistent Reduction In Risk Of Recurrence Across Key Subgroups Of Patients With Early Breast Cancer
Novartis will present late-breaking results from a prespecified exploratory subgroup analysis of invasive disease-free survival (iDFS) from the pivotal phase III NATALEE trial at the European Society for Medical Oncology (ESMO) Congress 2023. After 27.7 months of follow-up, the iDFS benefit with Kisqali (ribociclib) plus endocrine therapy (ET) was consistent across key prespecified subgroups, compared to ET alone, in patients with stage II and III hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) early breast cancer (EBC) (see table below)1. Results were also consistent with those in the overall trial population, reinforcing that the benefit was not driven by a specific patient subgroup.
"Subgroup analyses provide a more comprehensive picture of clinical benefit for patients and are critical to guiding treatment decisions, as they help indicate how different breast cancer subgroups might respond to treatment," said Aditya Bardia, M.D., Attending Physician, Medical Oncology, Mass General Cancer Center and Associate Professor, Medicine, Harvard Medical School and NATALEE trial investigator. "Given the outcomes of patients treated with endocrine therapy alone, this analysis outlines the potential benefit of adding ribociclib to endocrine therapy to reduce the risk of recurrence. These data provide important insight into how we think about residual risk in this population and make adjuvant treatment decisions for patients with localized breast cancer."
"Despite endocrine therapy, cancer recurrence remains unpredictable, and too many patients diagnosed with stage II or III HR+/HER2- early breast cancer experience their cancer coming back. This analysis further reinforces the potential of ribociclib to address the need for a new adjuvant option that reduces the ongoing risk of recurrence consistently across many types of at-risk patients," said Jeff Legos, executive vice president, global head of oncology development at Novartis. "These results from the NATALEE trial add to the wealth of efficacy, safety and quality of life data suggesting that ribociclib, if approved, could provide healthcare providers with a new option to help keep their patients living well and cancer-free."
Further analysis of the NATALEE trial is ongoing. Additional data, including the final efficacy analysis of the NATALEE trial, will be shared at upcoming medical meetings.
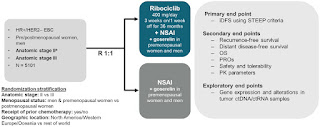
NATALEE is a global phase III multi-center, randomized, open-label trial to evaluate the efficacy and safety of ribociclib with ET as adjuvant treatment versus ET alone in patients with stage II and III HR+/HER2- EBC, being conducted in collaboration with TRIO2. The adjuvant ET in both treatment arms was a non-steroidal aromatase inhibitor (NSAI; anastrozole or letrozole) and goserelin if applicable. The primary endpoint of NATALEE is iDFS as defined by the Standardized Definitions for Efficacy End Points (STEEP) criteria. A total of 5,101 adult patients with HR+/HER2- EBC across 20 countries were randomized in the trial.
Results previously announced at the American Society of Clinical Oncology (ASCO) Annual Meeting 2023 showed ribociclib plus ET, compared to ET alone, lowered the risk of cancer recurrence by 25.2% (HR=0.748; 95% CI: 0.618, 0.906; p=0.0014), along with consistent clinically meaningful iDFS benefit across key pre-specified subgroups: AJCC Tumor Stage II (HR=0.761; 95% CI: 0.525, 1.103), AJCC Tumor Stage III (HR=0.740; 95% CI: 0.592, 0.925), node-negative disease (HR=0.630; 95% CI: 0.341, 1.165), node-positive disease (HR=0.771; 95% CI: 0.630, 0.944), pre-menopausal women and men (HR=0.722; 95% CI: 0.530, 0.983), post-menopausal women (HR=0.781; 95% CI: 0.613, 0.997)2. Ribociclib data across all secondary efficacy endpoints was also consistent, including distant disease-free survival (DDFS) (26% risk reduction) and recurrence-free survival (RFS) (28% risk reduction), with a trend for improvement in overall survival (OS) (HR=0.759; 95% CI: 0.539, 1.068).
For these previously announced results, median study duration of follow-up was 34 months (range 21-48 months) with clinical benefits observed after approximately two years2. NATALEE explored a lower starting dose (400 mg) of ribociclib than the dose approved for treatment in metastatic breast cancer (MBC) (600 mg) with the goal to minimize disruptions to patient quality of life without compromising efficacy. The safety profile of ribociclib at 400 mg was observed to have lower rates of symptomatic adverse events (AEs) and less need for dose modifications when administered up to three years. The most frequently reported AEs of special interest (grade 3 or higher) were neutropenia (43.8%) and liver-related AEs (e.G. Elevated transaminases) (8.3%).
More than 90% of patients diagnosed with breast cancer have EBC. Despite adjuvant ET, approximately one-third of those diagnosed with stage II and more than half of those diagnosed with stage III HR+/HER2- EBC experience cancer recurrence. The risk of recurrence continues over decades with more than half of breast cancer recurrences occurring five or more years after diagnosis. For many of these patients, there are currently no targeted therapeutic options outside of the standard chemotherapy and ET.
Kisqali has consistently demonstrated OS benefit while preserving or improving quality of life across three phase III trials in MBC. Updates to the NCCN Guidelines for breast cancer, released in January 2023, recommend ribociclib (Kisqali) as the only Category 1 preferred CDK4/6 inhibitor for first-line treatment of patients with HR+/HER2- MBC when combined with an aromatase inhibitor (AI). Additionally, Kisqali has the highest rating of any CDK4/6 inhibitor on the ESMO Magnitude of Clinical Benefit Scale, achieving a score of five out of five for first-line pre-menopausal patients with HR+/HER2- advanced breast cancer22. Further, Kisqali in combination with either letrozole or fulvestrant has uniquely, among other CDK4/6 inhibitors, received a score of four out of five for post-menopausal patients with HR+/HER2- advanced breast cancer treated in the first line.
Kisqali has been approved in 99 countries worldwide, including by the United States Food and Drug Administration (FDA) and the European Commission. In the US, Kisqali is approved for the treatment of adult patients with HR+/HER2- advanced or MBC in combination with an AI as initial ET or fulvestrant as initial ET or following disease progression on ET in post-menopausal women or in men. In the EU, Kisqali is approved for the treatment of women with HR+/HER2- advanced or MBC in combination with either an AI or fulvestrant as initial ET or following disease progression. In pre- or peri-menopausal women, the ET should be combined with a luteinizing hormone-releasing hormone agonist.
Novartis is committed to continuing to study Kisqali in breast cancer. Novartis is collaborating with SOLTI, which is leading the HARMONIA study to test whether Kisqali changes tumour biology to enable a better response to ET compared to Ibrance (palbociclib) for patients with HR+/HER2-, HER2-enriched subtype24 MBC, and with the Akershus University Hospital in Norway on the NEOLETRIB trial, a neoadjuvant phase II trial studying the effects of Kisqali in HR+/HER2- EBC to discover the potentially unique underlying mechanism of action.
Kisqali was developed by the Novartis Institutes for BioMedical Research (NIBR) under a research collaboration with Astex Pharmaceuticals.
Clinical Trials For Breast Cancer
Only about 3% of women with breast cancer take part in clinical trials, according to Y-ME, the national breast cancer organization.
This low level of clinical research participation may be stalling treatment progress. The fewer women who join clinical trials, the longer it takes to get data about whether a new treatment is an improvement over existing ones.
Should you join a breast cancer clinical trial? If you do, how can you choose the best one for you?
"Patients should be biased toward clinical trials," says Clifford Hudis, MD, Chief of the Breast Cancer Medicine Service at Memorial Sloan-Kettering Cancer Center in New York. "If you [are seen at a medical] center and they have a trial for which you're appropriate, you should seriously consider it."
What are the advantages of participating in a clinical trial?
Does that mean that there are no down sides to participating in clinical trials? Of course not. "Any time you're trying a new treatment or one with which we have less experience, there's always some potential for greater risk," says Eric Winer, MD, director of the Breast Program at Boston's Dana-Farber Cancer Institute.
Potential negatives include:
You may think that if you're not being treated at a major, nationally known cancer center such as Sloan-Kettering or Dana-Farber, you won't have the opportunity to participate in clinical trials. Not true.
"Many smaller community hospitals and cancer centers have trials available to them, either on their own or as part of larger cooperative groups," says Winer.
Before signing up for a clinical trial, find out as much as possible about what's involved. Here are some important questions to ask, according to Clinicaltrials.Gov -- a web site sponsored by the National Institutes of Health:
Some of your best sources for finding clinical trials:
Remember, the current "gold standard" treatments that you're benefiting from now wouldn't be available if other women hadn't joined clinical trials.
"I don't think we can say this enough. By participating in a trial, women with breast cancer are adding to knowledge that will help countless other women in the future," says Winer.
Breast Cancer News
Sep. 15, 2023 — The authors of new research led by the University of Exeter have warned that women who discover, outside of a clinical setting, that they carry a disease-causing variant in one of the BRCA genes ...
Sep. 12, 2023 — A review of COVID-19 studies globally has revealed reductions in breast cancer screening participation during 2020, with differences between geographic regions and healthcare ...
Sep. 11, 2023 — New research has found that estrogen receptor-positive breast cancer presents differing metabolic signatures in the blood of African American women and non-Hispanic white ...
Sep. 5, 2023 — Using a standardized assessment, researchers in the UK compared the performance of a commercially available artificial intelligence algorithm with human readers of screening ...
Sep. 4, 2023 — Scientists have discovered two new genes that cause head and neck cancer patients to be resistant to chemotherapy, and that silencing either gene can make cancer cells previously unresponsive to ...
Aug. 31, 2023 — The mechanism by which breast cancer is formed in the cells of mammalian epithelium has been discovered. Although roughly 20 mutations accumulate annually in each epithelial cell until menopause, the ...
Aug. 21, 2023 — Anti-estrogenic therapies can suppress the growth of cancer that does not express estrogen receptors; when combined with immune checkpoint inhibitor therapies, they halt tumor progression in mice ...
Aug. 18, 2023 — Women with larger breasts tend to exercise less frequently and avoid high-intensity exercise and a new study has found much improved participation in recreational group exercises after breast ...
Aug. 17, 2023 — Researchers have found some women with early-stage, low-risk breast cancer may not need radiotherapy after breast conserving ...
Aug. 9, 2023 — A collaborative study provides compelling evidence that combining an investigational oral drug with standard-of-care medications reverts hormone resistance and increases Rx effectiveness in ...
Aug. 3, 2023 — Chronic exposure to fine particulate air pollutants (PM2.5) and nitrogen dioxide (NO2) may increase non-lung cancer risk in older adults, according to new research. In a cohort study of millions of ...
Aug. 1, 2023 — Mammography screening supported by artificial intelligence (AI) is a safe alternative to today's conventional double reading by radiologists and can reduce heavy workloads for doctors. This has ...
July 31, 2023 — Scientists have identified a protein that plays a pivotal role in the action of several emerging cancer therapies. The researchers say the discovery will likely aid efforts to fine-tune the use of ...
July 28, 2023 — In hopes of improving the survival rate for breast cancer patients, researchers designed a wearable ultrasound device that could allow women to detect tumors when they are still in early ...
July 21, 2023 — Scientists have discovered a possible way to block proteins produced in the body when a patient has cancer and which causes its spread to other parts of the ...
July 19, 2023 — Women diagnosed and treated for breast cancer have increased biological aging compared to women who remain free of breast cancer, according to a new study. Among women diagnosed with breast cancer, ...
June 28, 2023 — A new study has created the world's largest and most comprehensive map of normal breast tissue, providing an unprecedented understanding of mammary biology that may help identify therapeutic ...
June 21, 2023 — Researchers have outlined the structure and function of a protein complex which is required to repair damaged DNA and protect against ...
May 17, 2023 — Researchers trace the origin of certain breast cancers to genomic reshuffling -- rearrangement of chromosomes -- that activates cancer genes and ignites disease. The finding offers a long-missing ...
May 4, 2023 — Researchers discover a mechanism by which cancer cells escape the immune ...
Friday, September 15, 2023
Tuesday, September 12, 2023
Monday, September 11, 2023
Tuesday, September 5, 2023
Monday, September 4, 2023
Thursday, August 31, 2023
Monday, August 21, 2023
Friday, August 18, 2023
Thursday, August 17, 2023
Wednesday, August 9, 2023
Thursday, August 3, 2023
Tuesday, August 1, 2023
Monday, July 31, 2023
Friday, July 28, 2023
Friday, July 21, 2023
Wednesday, July 19, 2023
Wednesday, June 28, 2023
Wednesday, June 21, 2023
Wednesday, May 17, 2023
Thursday, May 4, 2023
Wednesday, March 22, 2023
Thursday, March 16, 2023
Monday, March 13, 2023
Wednesday, March 8, 2023
Wednesday, February 22, 2023
Tuesday, February 7, 2023
Wednesday, January 11, 2023
Thursday, December 8, 2022
Monday, December 5, 2022
Thursday, December 1, 2022
Monday, November 21, 2022
Friday, November 18, 2022
Thursday, November 17, 2022
Tuesday, November 15, 2022
Friday, November 11, 2022
Thursday, November 10, 2022
Wednesday, November 9, 2022
Monday, November 7, 2022
Thursday, November 3, 2022
Monday, October 31, 2022
Friday, October 28, 2022
Thursday, October 27, 2022
Tuesday, October 25, 2022
Friday, October 21, 2022
Tuesday, October 18, 2022
Monday, October 17, 2022
Friday, October 14, 2022
Friday, September 30, 2022
Friday, September 23, 2022
Thursday, September 22, 2022
Tuesday, September 20, 2022
Wednesday, September 14, 2022
Tuesday, September 13, 2022
Monday, September 12, 2022
Thursday, September 1, 2022
Wednesday, August 31, 2022
Tuesday, August 30, 2022
Friday, August 26, 2022
Thursday, August 25, 2022
Wednesday, August 24, 2022
Tuesday, August 16, 2022
Wednesday, August 10, 2022
Monday, August 8, 2022
Wednesday, July 27, 2022
Thursday, July 21, 2022
Tuesday, July 19, 2022
Monday, July 18, 2022
Monday, June 27, 2022
Thursday, June 23, 2022
Wednesday, June 22, 2022
Tuesday, June 14, 2022
Thursday, June 9, 2022
Friday, June 3, 2022
Thursday, June 2, 2022
Tuesday, May 31, 2022
Monday, May 30, 2022
Tuesday, May 24, 2022
Tuesday, May 17, 2022
Thursday, May 12, 2022
Tuesday, May 10, 2022
Monday, April 25, 2022
Sunday, April 24, 2022
Friday, April 22, 2022
Thursday, April 21, 2022
Monday, April 11, 2022
Thursday, April 7, 2022

Comments
Post a Comment